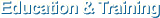About Web-based Clinical Trial Management System (eVelos System)
eVelos system is Web-Based Clinical Trial Management System developed by Velos Inc.(USA) and certified by NCI’s Bronze Lever (CDE, CTC, AE, report, caBIG affinity). Anywhere internet available can be the spot to use it and one-stop-service is provided from clinical service design to data management and statistics analysis.
Especially, effective clinical research became enabled with this system convenient to integrate information management among researchers, clinical test requester, monitor, and subject while carrying out multi-organization and multi-national researches.
In order to achieve objectivity and reliability of clinical research data, American standard (21 CFR Part 11) was observed. Major cancer centers and institutes in U.S.A are using eVelos System. (About 40% of group supported by NCI)
Security of eVelos System
- National Cancer Center endeavors to strengthen the security by providing the vaccine server installation, firewall installation to block external connection, intrusion protection system, web service operational separation, public key infrastructure (PKI), and regular DB Backup..
- Users with complete identification are allowed to use the system because Accredited Certificate would also require in addition to ID/Password when user tries to log-on. (21 CFR Part 11.10 & 11.200 followed)
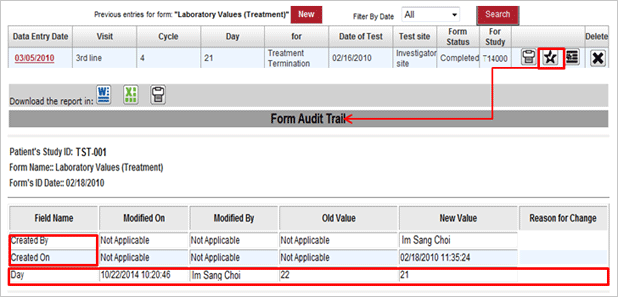
- Audit menu located at each upper CRF automatically records, upon occurrence, such as users to input CRF, modified time and value, and information of users modified. (21 CFR Part 11.10 followed)
Necessity
- Size of domestic/overseas clinical research has been increased and multi-national pharmaceutical companies’ investments on such research also increased accordingly
- Necessity of eVelos System which supports from planning, execution, analysis, and reports of systemic clinical research at national standard has increased to improve objectivity and reliability of the clinical research.
- Necessity to assure the foundation for carrying out clinical research & its data collection and monitor the quality of such research in accordance with international regulation of clinical research has been increased
- An operation system which enables centralized data management to play a leading role in the clinical research at multi-organization
Background of local introduction and its application
National Cancer Center firstly introduced eVelos System Mar 2007 in Asia.
| 2007.03 | Made contract with Velos (7 years) |
|---|---|
| 2007.05 | Participated eVelos System user conferences & workshop (San Francisco) |
| 2007.06 | Held eVelos System introduction workshop (National Cancer Center) |
| 2007.08 | Trial run of eVelos System test version |
| 2007.11 | Ran eVelos System operation version |
| 2007.11 | Opened intensive training and regular training course by each subject of eVelos System |
| 2007.12 | Started multi-organization clinical research |
| 2008.04 | Provided Accredited Certificate service |
| 2008.05 | Participated eVelos System user meeting (San Francisco) |
| 2008.08 | Developed web-based random assignment function |
| 2009.01 | eVelos System Version Upgrade (ver. 8) |
| 2009.04 | Developed real-time alarm function in case of web-based random assignment |
| 2009.05 | Participated to eVelos System user meeting and announced usage status of National Cancer Center (San Francisco) |
| 2009.08 | Transferred the organization to Office of Clinical Research Coordination (Under the direct control of National Cancer Center) |
| 2009.09 | Held eVelos System workshop (National Cancer Center) |
| 2009.10 | Provided e-Learning training program for eVelos System) |
| 2010.05 | Participated eVelos System user meeting (San Francisco) |
| 2010.10 | Held ‘Electronic Data Capture & Data Interface for Clinical Research’ Workshop |
| 2012.05 | Velos annual user meeting |
| 2012.06 | Development of Auto Calendar/ Patient Study Status Association Function |
| 2012.10 | Workshop:Systematically build & Promoting of Multi-cetrial |
| 2013.05 | Velos annual user meeting |
| 2013.10 | Update the e-Learning education program |
| 2014.04 | Velos annual user meeting |
| 2014.08 | eVelos System Version Upgrade (ver.9) |
| 2014.12 | Velos & SWOG formal visit |
| 2015.03 | License agreement with Velos (7 years) Workshop:Velos System - Future Plans and Strategies |
| 2015.11 | 4th Clinical Research Coordination Center NCC Workshop |
| 2016.04 | Velos annual user meeting |
| 2016.11 | License Agreement20150307 Addendum#1_PT(Present Tense) with Velos |
Overseas (America) background and its application
Recently, there is effort made to standardize all CRF of clinical researches that are sponsored by NCI and use of eVelos System as part of effort is suggested. 9 out 18 top class cancer centers and 12 research centers out of 19 News Best Hospitals awarded 2007 in America are using eVelos System. For such a reason, users of eVelos System are rapidly increasing and about 40% of group supported by NCI now are using the system.
Main organization users
| Organizations | |
|---|---|
| MD Anderson | Johns Hopkins |
| UCLA Medical Center | Cleveland Clinic |
| Memorial Sloan Kettering | University of Michigan |
| Duke | U. of Pennsylvania |
| University of Chicago | New-York Presbyterian |
| UCSF | Washington University |
| Cedars Sinai | Yale-New Haven |
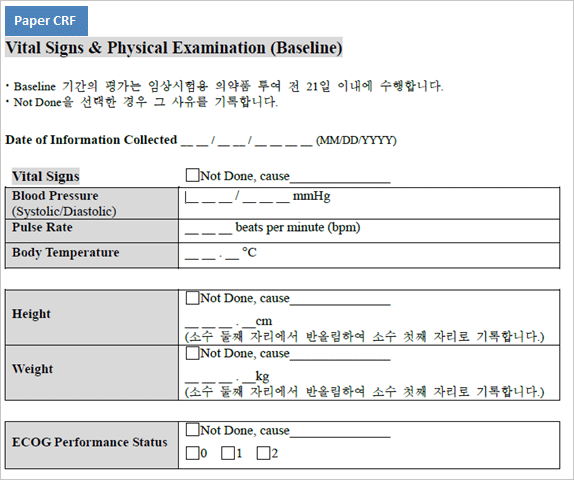
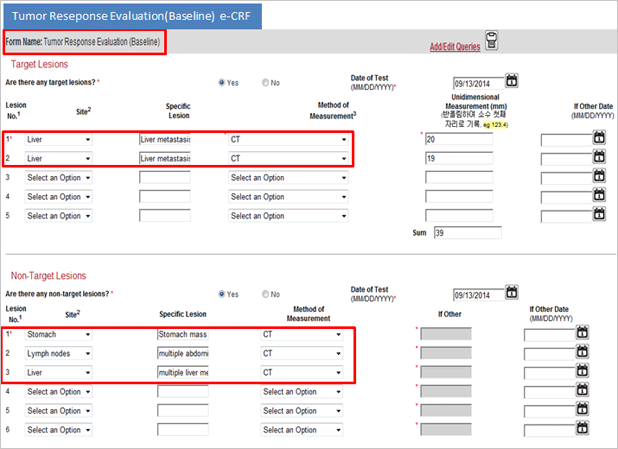
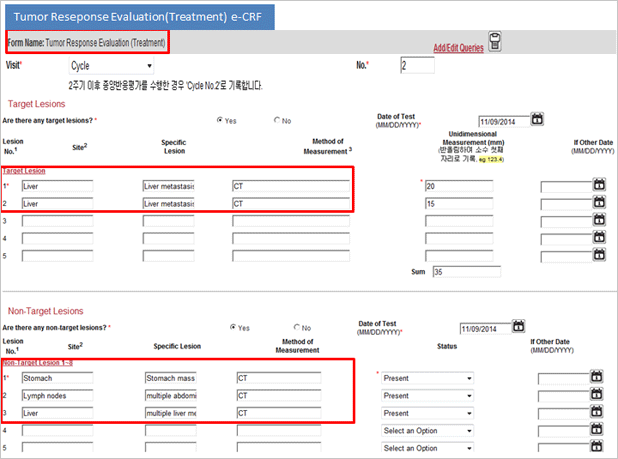
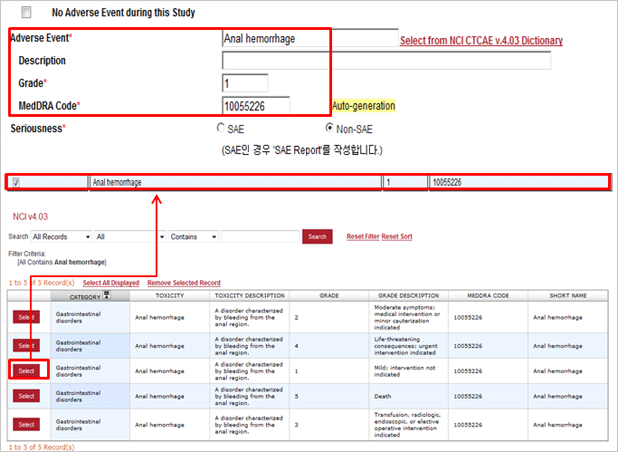
Difference between Paper CRF and eVelos System’s e-CRF
| Paper CRF | Electronic CRF (e-CRF) |
|---|---|
| Data re-entry to Excel is required after data filled in paper CRF. | Data becomes automatically saved in DB upon data entry to e-CRF. |
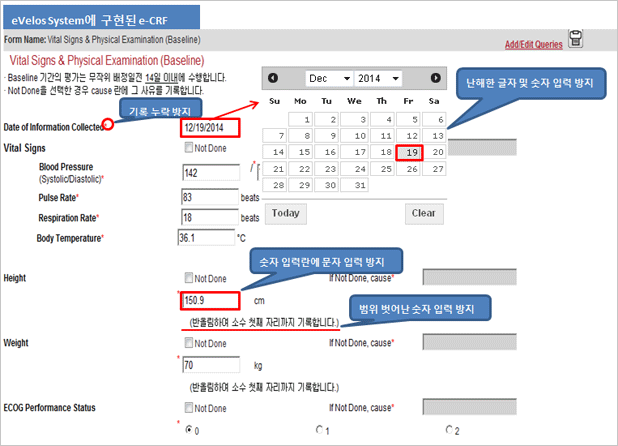
| May have entry errors such as text entered to number entry filed. Example) number of times in 5 years: Few times. |
Save function becomes disabled when text is entered because of the text entry protection into number entry field function. |
| May have essential items missing | Protects against the record missing by blocking save function if critical items are not entered. (Mandatory Field Setup) |
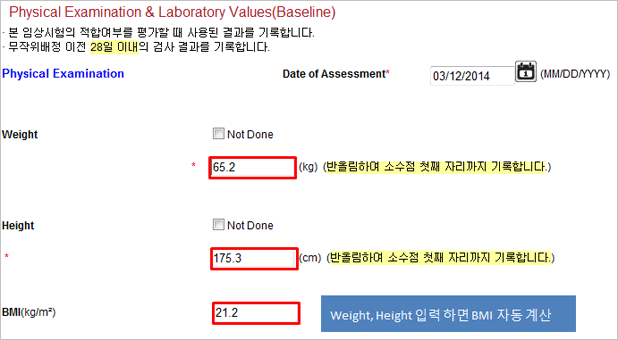
| May enter value out of range. (Age, height, weight) Example) age: 200 years old |
Out of range number entry is protected. |
| May have illegible letters written in hands. | No illegible letters due to electronic entry |
| May have wrong value due to manual calculation in case of calculated value entry | Protects against the calculation error with automatic scoring and calculation by items selected. |
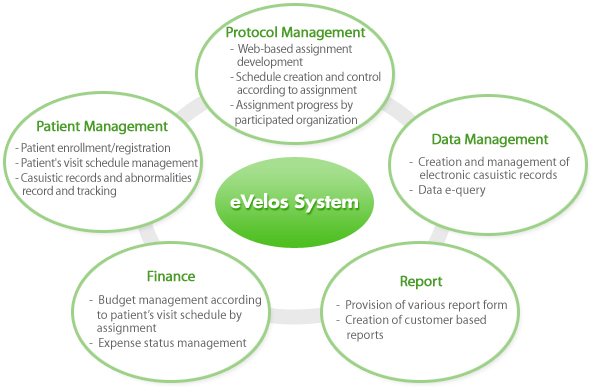
Main functions
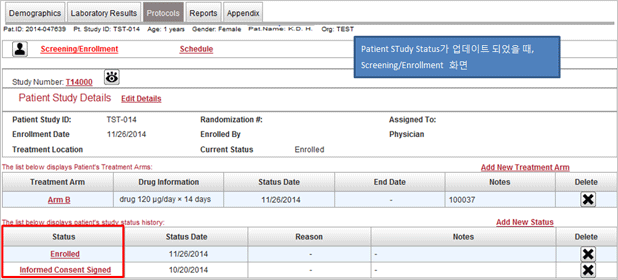
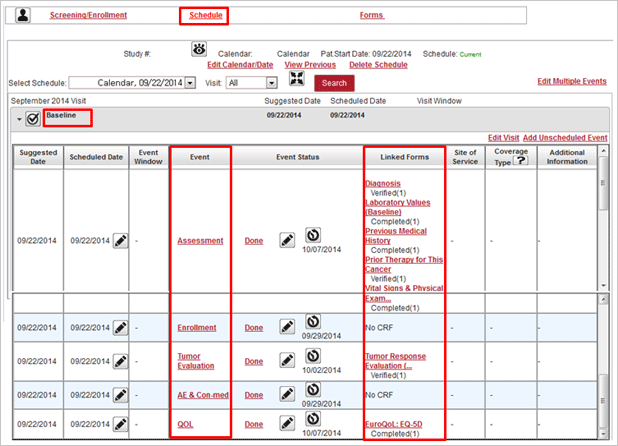
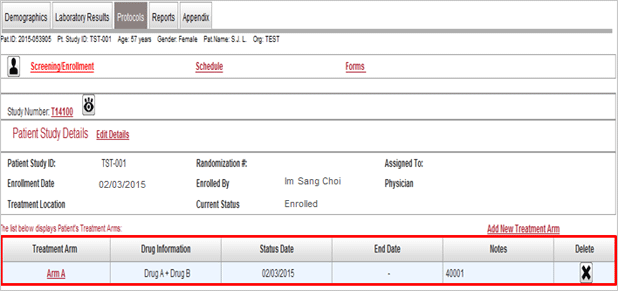
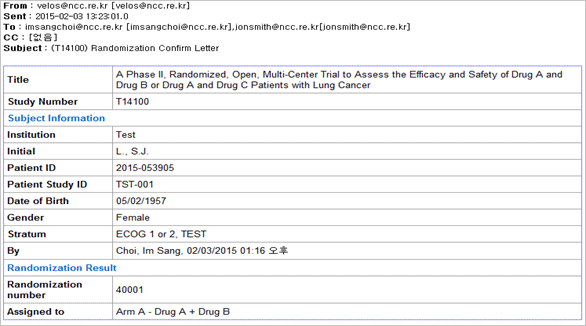
Protocol management
- This menu enabled many researchers’ ideas are collected and shared for protocol development by using
website from the very beginning when researchers have study ideas.
- Management and sharing of version of study relevant materials
- CRF by assignment, schedule, random assignment and risk management setup
- Controls progress of assignment by organization and IRB status
Study team registration and system use permission management
This menu provides team member to be registered by organizations that participate into each assignment
and different access control according to their role are given.