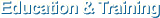The National Cancer Center (NCC) of Korea launched a new government funded R&D program, called NOIU for cancer drug development in 2011 to bridge the gap between discovery and development in drug development in Korea. Main objective of NOIU is to co-develop new anti-cancer drug candidates from the original inventors in academia, government-funded institutes and industry by a group of drug development experts in NOIU carrying out preclinical and early clinical trials. The National Oncoventure aims to develop 4 Global Drug Candidates that will have completed phase 2 human clinical trials by 2025.
Pipeline
○ current Pipeline as of May 2021
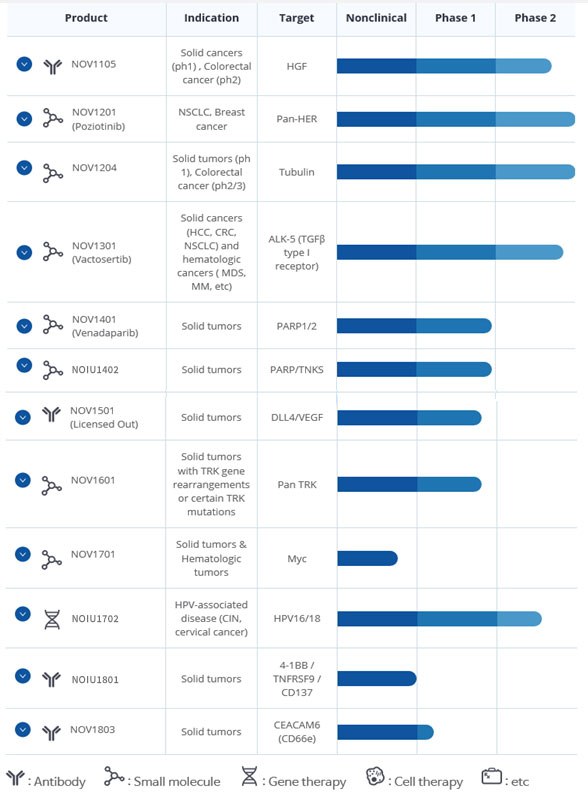
- NOV 1105, Solid Cancers(ph1), Colorectal cancer(ph2)
- NOV1201, (Poziotinib)NSCLC, Breast cancer
- NOV1204, Solid tumors (ph 1), Colorectal cancer (ph2/3)
- NOV1301(Vactosertib), Solid cancers (HCC, CRC, NSCLC) and hematologic cancers(MDS, MM, etc)
- NOV1401(Venadaparib), Solid tumors
- NOIU1402, Solid tumors
- NOV1501 (Licensed Out)Solid tumors
- NOV1601, Solid tumors with TRK gene rearrangements or certain TRK mutations
- NOV1701, Solid tumors & Hematologic tumors
- NOIU1702, HPV-associated disease (CIN, cervical cancer)
- NOIU1801, Solid tumors
- NOV1803, Solid tumors
- NOV 1105, Solid Cancers(ph1), Colorectal cancer(ph2)
- NOV1105 is an anti-hepatocyte growth factor (HGF) antibody effectively down-regulating the signaling HGF/c-Met pathway. This Humanized IgG4 with a KD of 1.2 x 10-10 M demonstrates excellent safety and PK profiles. In vivo studies show tumor growth inhibition and increased survival in Glioblastoma Multiforme (GBM), soft tissue sarcoma (STS) and ovarian cancer models alone or in combination with other drugs. With delicate roles of HGF in the tumor microenvironment and with recently found novel biomarkers strongly related with HGF, the clinical POC of first in human is expected to not only uncover the optimal population of responders but also to give us the more clues for HGF in tumorigenesis. We completed Phase 1 studies for solid tumors(MFDS) with several cases of stable diseases and partial responses* and have started to enroll the patients for phase 2 study for colorectal cancers this Summer 2019.
- * First-in-human phase I trial of anti-hepatocyte growth factor (HGF) antibody (YYB101) in refractory solid tumor patients: Integrative pathologic-genomic analysis and the final results. ASCO poster 2019
-
NOV 1105, Solid Cancers(ph1), Colorectal cancer(ph2) Target Hepatocyte Growth Factor (HGF) MOA Inhibition of Hypoxia-induced HGF – cMET signaling by anti-HGF antibody Dev. Stage Phase 2 Profile Humanized anti-HGF antibody(IgG4), No CDC, ADCC effect. Affinity 3.6pM, Half Life 21 days in Monkey, 11 days in mouse 4 weeks repeated Tox: NOAEL 200mpk, well tolerated Efficacy Potent inhibition in in vitro /in vivo model of Glioblastoma and Leiomyosarcoma Dosing IV, infusion, once every 2 weeks Indication – Solid tumors including Melanoma, NSCLC, CRC, Sebaceous carcinoma (phase 1)
– CRC in combination with Irinotecan (Phase 2)Competition Ficlatuzumab (Phase II) Originator CellabMED
- NOV1201, (Poziotinib)NSCLC, Breast cancer
- Poziotinib (NOV120101) is an oral, irreversible inhibitor of EGFR, HER2 and HER4. Preclinical studies conducted in cell lines and xenograft models of NSCLC revealed that Poziotinib has more potent activity than gefitinib, erlotinib and even afatinib in lung cancer models with activating EGFR mutations or T790M mutation. A phase I study to investigate the safety and maximum tolerated dose (MTD) of Poziotinib in genetically-unselected patients with advanced solid cancers including NSCLC and Breast cancer showed that 14% (7/51) of patients experienced partial response (PR), with the MTD of 24 mg once daily and acceptable toxicity profile, supporting further clinical development of Poziotinib. In phase II open-label, single-arm study was conducted to explore the anti-cancer activity and safety of Poziotinib in patients with advanced or metastatic lung adenocarcinoma with activating EGFR mutations, who developed acquired resistance (AR) to EGFR TKIs based on the Jackman criteria. A Prospective, Open-label, Single-arm, Multi-Center, Phase II Trial of NOV120101 in Patients With HER2-overexpressed Recurrent Stage IV Breast Cancer Who Have Received at Least 2 Prior HER2-directed Regimens is now in progress. It was licensed out to Spectrum Pharmaceuticals for global development in 2015.
-
NOV1201, (Poziotinib)NSCLC, Breast cancer Target pan-HER receptor MOA Inhibition of HER signaling Dev. Stage Phase 2 Profile Humanized anti-HGF antibody(IgG4), No CDC, ADCC effect. Affinity 3.6pM, Half Life 21 days in Monkey, 11 days in mouse 4 weeks repeated Tox: NOAEL 200mpk, well tolerated Efficacy in phase 1, response rate was 20% in solid tumors and overall response rate was 60% in breast cancers Dosing PO Indication Breast cancer, NSCLC Competition afatinib,neratinib Originator Hanmi Pharmaceuticals Licensee(Collaborators) Spectrum Pharmaceuticals, Inc.
- NOV1204, Solid tumors (ph 1), Colorectal cancer (ph2/3)
- NOV1204 (CKD-516) is a tubulin inhibitor under development for the treatment of advanced solid tumors (ph1,IV and oral) and colorectal cancer (ph1b/2a on going, pivital in plan, oral form). NOV1204 is a benzophenone derivative and water-soluble valine prodrug which binds to tubulin and prevents its polymerization in the tumor blood vessel and tumor cells (vascular disrupting agent: VDA). It blocks the formation of the mitotic spindle and leads to cell cycle arrest at the G2/M phase. As a result, this agent not only disrupts the tumor vasculature and tumor blood flow, deprives tumor cells of nutrients and induces tumor cell apoptosis, but also has a direct cytotoxic effect on tumor cells by inhibiting tubulin polymerization. In vivo studies showed tumor inhibition and tumor regression, alone or in combination, while demonstrating good safety and PK profiles. Having a wider therapeutic margin and good bioavailability, NOV1204 is expected to be the only market drug of the VDAs which can be administrated orally with little concern about cardiovascular toxicity issue. These characteristics make it possible to design the patient-friendly dosing plan of daily oral low dose. It was proven to have efficacy in colorectal cancers in combination with irinotecan at phase 1b. With the promising result from phase 1b, We are planning to expand this study as a pivotal study in the 2nd half of this year 2019.
Currently, a lot of proofs indicate that clinical outcomes have to do not only with the main characteristics of vascular disruption but also with its immune-boosting ability, one of the unique features of NOV1204. It was shown to increase DC maturation, antigen presentation, CD8+ T cell proliferation, and tumor infiltration in animal models. In syngeneic mice model, NOV1204 had the synergistic effect in combination with anti-PD-1, PD-L1 or CTLA-4 antagonists. Being an oral agent, flexible dosing schedule optimization is possible. -
NOV1204, Solid tumors (ph 1), Colorectal cancer (ph2/3) Target Tubulin polymerization MOA Vascular disrupting agent Dev. Stage –Phase 3 IND approved in Korea(Combination study with irinotecan in CRC) Profile – An orally active potent small-molecule inhibitor
– Excellent aqueous solubility
– Excellent PK w/ good oral bioavailabilityEfficacy – Good anticancer effects in various solid tumors including advanced drug-resistant tumors
– Suitable for combination therapy (with Chemo, With IO)Dosing PO (5d on / 2d off) Indication Solid tumors Competition Plinabulin (Ph3), phosbretabulin(CA4P,Ph3), BNC-105P(Ph2) Originator CKD Pharm.
- NOV1301(Vactosertib), Solid cancers (HCC, CRC, NSCLC) and hematologic cancers(MDS, MM, etc)
- NOV1301 (TEW-7197) is an ALK5 (TGF ß type I receptor) inhibitor under development for the treatment of solid tumors and CML. It reduces the signaling of TGF ß, typically increased during cancer by inhibiting its receptor ALK5, whilst normalizing the tumor microenvironment by modulating the extracellular matrix, allowing angiogenesis and enhancing immune surveillance. In vivo it reduced metastasis and inhibited tumor growth in breast, melanoma, HCC, GBM and CML models, leading to extended survival. NOV1301 exhibits good safety and PK profiles in an oral once a day dosing.
- It is at the completion stage of phase 1 FIH under the US FDA IND approval. We are actively enrolling patients to several phase 2 studies.
- Currently the biomarker study is ongoing for patient selection strategy. It is based on the status of the TBRS (TGF Beta Response Signature), which will show us who will get benefit from this drug.
-
NOV1301(Vactosertib), Solid cancers (HCC, CRC, NSCLC) and hematologic cancers(MDS, MM, etc) Target TGFβ type I receptor(ALK5) inhibitor MOA Inhibits EMT and metastasis & activates immune surveillance Dev. Stage Phase 2 (US, KR) Profile – An orally active potent small-molecule inhibitor
– Potent & selective
– Good ADME & PK profileEfficacy – Good efficacy in various in vivo animal models
– Suitable for combination therapyDosing PO Indication – Solid tumors (Breast, Melanoma, HCC, GBM at Phase1)
– Hematologic tumors (MM, MDS, CML at phase 2)
– CRC in combination with Keytruda (phase 2)
– NSCLC in combination with Imfinzi (phase 2)
– Gastric Cancer in combination with Paclitaxel (phase 2)
– Pancreatic Cancer in combination with FOLFOX (phase 2)
– Desmoid sarcoma in combination with Imatinib (phase 2)Competition Galunisertib (Phase 3) Originator MedPacto Inc.
- NOV1401(Venadaparib), Solid tumors
- NOV1401 selectively binds to and inhibits ADP-ribose polymerase-1 and 2, inhibiting PARP-mediated repair of single-strand DNA breaks leading programmed cell death. It inhibits PARP-1,2 in nanomolar levels and shows greater anti-tumor effects than the other market PARP inhibitors in various cell lines and animal models with BRCA mutations and HRD. Upon confirmation of its clinical feasibility at phase 1, parallel clinical-development of companion diagnostics will be initiated using biomarkers for prognostic and monitoring purpose for this drug responses.
-
NOV1401(Venadaparib), Solid tumors Target Poly (ADP-ribose) polymerase-1 (PARP-1) inhibitor MOA Inhibition of PARP-mediated repair of single-strand DNA Dev. Stage Phase 1 trial in Korea is ongoing Profile – An orally active potent small molecule inhibitor
– Excellent aqueous solubility
– Potent & selective
– Good ADME & PK profileEfficacy – Good efficacy in various in vivo animal models
– Suitable for combination therapyDosing PO Indication Solid tumors Competition Olaparib, Rucaparib, Niraparib, Talazoparib (Ph III) Originator ILDONG Pharmaceuticals Inc.
- NOIU1402, Solid tumors
- NOV1402 acts by targeting both poly ADP-ribose polymerases (PARPs) and tankyrase (TNKS). PARP is a DNA nick-sensor that signals the presence of DNA damage and facilitates DNA repair. Inhibition of PARP increases the activity of DNA damaging agents and causes cancer cell death. Inhibition of tankyrase not only induces interruption of the Wnt/β-catenin signaling by stabilizing axin and promoting β-catenin degradation but prevents non-homologous end joining (NHEJ) activation that repairs double-strand breaks in DNA, which lead to prevention of tumor growth. NOV1402 is expected to be superior to olaparib or a similar class of PARP inhibitors in its efficacy and to talazoparib in its toxicity based on its dual mode of actions and physicochemical properties. Upon confirmation of its clinical feasibility, parallel development of companion diagnostics is planned.
-
NOIU1402, Solid tumors Target Poly (ADP-ribose) polymerase-12,3,4 and tankyrase MOA Inhibition of PARP-mediated repair of single strand DNA and Wnt/b-catenin signaling Dev. Stage Phase 1b trial in Korea is ongoing in a basket trial recruiting HRD positive tumor patients regardless of tumor sites. Profile – An orally active potent small-molecule inhibitor
– Excellent aqueous solubility
– Potent & selectiveEfficacy Good efficacy in various in vivo animal models Dosing PO, Q.D Indication Solid tumors Competition PARP inhibitors (Olaparib, Rucaparib, Niraparib), TNK inhibitors(G007-LK,JW-55,XAV-939, NVP-TANKS656) Originator Jeil Pharmaceuticals Inc.
- NOV1501 (Licensed Out)Solid tumors
- VEGF is a key inducer of angiogenesis in cancer, forms new blood vessels and results in tumor growth beyond a certain size. Anti-VEGF therapy has been widely used in patients with various tumor types, but the effects are variable and resistance is frequently encountered. VEGF induces DLL4 expression in endothelial tip cells. VEGF-induced expression of DLL4 in vascular endothelium leads to the activation of Notch signaling. Blocking the Notch/DLL4 signaling results tumor growth due to the formation of immature and poorly functional vessels that result in reduced tumor perfusion. Blockade of DLL4 can have potent inhibition effects on tumor growth that are resistant to anti-VEGF therapies. Furthermore, the simultaneous targeting of DLL4 and VEGF has produced additive of synergistic anti-tumor effects compared to single agents in a number of tumor models.
NOV1501 is an anti-DLL4/anti-VEGF bispecific monoclonal antibody. It inhibits both DLL4/Notch and VEGF/VEGFR2 interactions in nanomolar level. It shows greater anti-cancer effects in various animal models in comparison to anti-DLL4 or anti-VEGF agents. NOV1501’s unique design of the bispecific antibody helps simpler purification and higher anti-cancer efficacy. -
NOV1501 (Licensed Out)Solid tumors Target DLL4 and VEGF MOA Anti-angiogenesis by inhibiting DLL4/Notch and VEGF/VEGFR2 interaction Dev. Stage Phase 1 (FIH) Profile – Unique design bispecific Antibody
– Good synergy in vivo
– Active in Avastin-resistant tumorsEfficacy – Good efficacy in various in vivo animal models
– Suitable for combination therapyDosing IV infusion, Q2W Indication Solid tumors (Gastric Cancer, Lung, Colon) Competition Navicixizumab (Ph1), ABT-165 (Ph 1) Originator ABL Bio, Inc. Licensee TRIGR pharmaceuticals
- NOV1601, Solid tumors with TRK gene rearrangements or certain TRK mutations
- NOV1601(CHC2014) selectively inhibits not only pan-TRK(TRKA, TRKB, and TRKC), but also mutated forms of TRKAs(G595R & G667C). TRK is known to be involved in several diseases, and especially TRK gene fusion has been reported in many types of cancers. NOV1601 blocks the activity of tyrosine kinases and the signaling pathways they activate, thereby exhibiting the therapeutic effect. NOV1601 is currently on the phase 1 FPI trial. Identification of TRK gene fusion will be performed using the companion diagnostic platforms that are under development.
-
NOV1601, Solid tumors with TRK gene rearrangements or certain TRK mutations Target Pan-TRK MOA TRK 의 타이로신 카이네이즈 활성과 관련 신호전달경로를 억제 Dev. Stage Phase 1 Profile – An orally active potent small molecule inhibitor
– Potent & selective
– Good ADME & PK profile
– Parallel Development of companion diagnostics for TRK detectionEfficacy – Good efficacy in various in vivo animal models Dosing PO, QD Indication Solid tumors with TRK gene rearrangements or certain TRK mutations Competition Larotrectinib (market), Loxo-195 (Ph2) Originator Handok, CMG Pharma
- NOV1701, Solid tumors & Hematologic tumors
- NOV 1701 is a synthetic compound program that directly inhibits MYC. Unlike many other Myc inhibitors that have been developed so far, NOV1701 has a new mechanism that directly inhibits the binding of Myc / Max dimers to DNA. In other words, other c-Myc inhibitors indirectly inhibit Myc by modulating the universal Leucine-Zipper protein PPI of the surrounding regulators, while NOV1701 specifically and directly inhibits Myc, a substantial target protein. While the attempt to develop other platform materials (siRNA, peptide) or synthetic compounds that were under development as Myc direct inhibitors has not been very successful, NOV1701 has been proved to be the only one with high selectivity, efficacy and safety (in vivo).
-
NOV1701, Solid tumors & Hematologic tumors Target Myc MOA Myc/Max dimer 가 DNA 에 결합하는 것을 직접적으로 저해 Dev. Stage Non GLP development Profile first in class Myc direct inhibitor Efficacy Good efficacy in several cancer models (Lung, Colon, etc) Indication Solid tumors and Hematological tumors (Lymphoma, etc) Competition -Direct inhibitor: AVI-4126 (ph II/III)
-Indirect inhibitor: TEN-10 (ph IB), OTX015 (ph IB)Originator National Cancer Center and Korea Research Institute of Chemical Technology
- NOIU1702, HPV-associated disease (CIN, cervical cancer)
- NOV1702 (GX-188E) is a therapeutic DNA vaccine, in the form of a plasmid that codes the E6 and E7 oncoproteins of HPV 16 and 18 types. It enhances the immune response by simultaneously expressing tPA for the extracellular secretion of expressed E6/E7 and Flt-3L, which promotes dendritic cells and its uptake of these antigens. When DNA injected by electroporator produces E6 / E7 protein, the human body induces the cytotoxic T lymphocyte response to tumor cells that express E6/E7 oncoprotein.
We are doing phase 1b/2a study for cervical cancer in combination with anti-PD1 antibody pembrolizumab based on the result of the phase 1 study where 8 out of 9 CIN3 patients showed an enhanced antigen-specific immune response and 7 of them had a complete loss of lesion and removal of HPV 16 or 18 types,* expecting its anti-cancer immune response to cervical cancer can be enhanced if we prevent CD8+ T Cell exhaustion by PD-1 Immune checkpoint inhibition(NCT03444376). - * Kim TJ et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun. 2014 Oct 30;5:5317
-
NOIU1702, HPV-associated disease (CIN, cervical cancer) Target E6 and E7 of HPV 16, 18 type MOA Promoting a tumor-specific, CD8+ immune response against HPV E6 and E7 epitopes through vaccination (GX-188E) Dev. Stage Phase 1b/2 started in 2018 (A Multi-Center, Open-label Phase Ib-II Trial of the Combination of GX-188E Vaccination and Pembrolizumab in Patients With Advanced, Non-Resectable HPV Type 16 and/or 18 Positive Cervical Cancer_NCT03444376) Profile 6,085 base pair plasmid DNA vaccine containing E6/E7 genes of HPV16 and 18, fused to Flt3 (Fms-like tyrosine kinase 3) ligand Efficacy Therapeutic potential (regression of lesions and viral clearance) in CIN3 patients Dosing Intramuscular administration Indication HPV-associated cervical cancer Competition VGX-3100(INOVIO, Phase III), ADXS11-001(Advaxis, Phase II-III) Originator Genexine Inc.
- NOIU1801, Solid tumors
- 4-1BB is a receptor of T lymphocyte and its signal is required for proper T cell activation. NOV1801 is an anti-4-1BB therapeutic monoclonal antibody. Co-stimulatory receptor, 4-1BB is not expressed in resting T cells and is up-regulated following activation. In other words, 4-1BB is induced on CD8+ T cell upon stimulation and generate proliferation and anti-apoptotic signaling with ligation of NOV1801. In addition, agonistic anti-4-1BB antibody NOV1801 induce secretion of INF-gamma which plays a pivotal role in promoting immune reaction against primary and metastatic tumors. In the study of humanized mice that possess the human immune system, NOV1801 eradicate the human tumor and show synergistic anti-tumor activity with immune checkpoint blockade. Thus, NOV1801 represent diverse advantages in anti-cancer therapy;
- Excellent anti-tumor efficacy
- Minimize adverse event of cancer therapy
- Inhibit recurrence of a tumor through the memorized immune system
- Synergize anti-cancer effect with immune checkpoint modulator or chemotherapy
-
NOIU1702, HPV-associated disease (CIN, cervical cancer) Target 4-1BB/TNFRSF9/CD137 MOA Activation of cytotoxic CD8+ T cell Dev. Stage Preclinical, IND for phase I planned for 2020 Profile Humanized anti-human 4-1BB antibody. KD: 7.975E-10 (M) Efficacy Excellent anti-tumor efficacy in humanized mice model Dosing I.V. Indication Solid tumors Competition Urelumab (BMS, phase I or II), Utomilumab (Pfizer, phase I or II) Originator Eutilex Co., Ltd.
- NOV1803, Solid tumors
- NOV1803 (DNP002) is a humanized anti-CEACAM6 antibody. It can bind and destroy both cancer cells and MDSC. NOV1803’s epitope is B domain of CEACAM6, therefore it does not block the CEACAM1 and CEACAM6 interaction. Rather, by binding the membrane-proximal region of CEACAM6, NOV1803 strongly induces ADCC. Ex vivo studies with advanced gastric cancer (AGC) patients showed the almost complete depletion of cancer cells following the activation of autologous immune cells. Besides, NOV1803 effectively depleted the MDSC (myeloid-derived suppressor cells) in Ex Vivo studies using the whole blood of AGC patients. Also, strong tumor growth inhibition was shown in several mouse models including NSCLC, gastric cancer, and colon cancer.
In terms of mode of action of NOV1803, it was confirmed that NOV1803 induced not only the NK cells activation but also the activation of T cells and other immune cells. In addition to the ability to remove MDSC, induction of various immune effector cell activation is expected to lead to the overall activation of the immune system, especially in the tumor microenvironment, and thus influence positively the anticancer effect of NOV1803.
ND for Phase I study approved in September 2020. -
NOIU1702, HPV-associated disease (CIN, cervical cancer) Target CEACAM6 (CD66c) MOA Dual targeting (CEACAM6 + tumor and MDSC)Immune boosting by NK cell activation by enhanced ADCC Dev. Stage Preclinical, IND for phase I planned for 2020 Profile Humanized anti-CEACAM6 antibody(IgG1), Affinity KD 6.64E-10 (M),
MCB established; Ab. Titer > 4.5 g/LEfficacy Good efficacy in various in vivo animal models,
Tumor cell killing by autologous immune cells of Gastric cancer patients in Ex vivo.Dosing I.V., infusion, once every weeks Indication Solid tumors (NSCLC, gastric cancer, colon cancer) Competition NEO-201 (Precision Biologics, Phase I), BAY 1834942 (Bayer, Phase I) Originator DINONA Inc.